Sacroiliac Joint Issues in Pediatrics Continuing Education
- Research article
- Open Access
- Published:
Anatomy of the sacroiliac joints in children and adolescents by computed tomography
Pediatric Rheumatology volume 15, Article number:82 (2017) Cite this article
Abstract
Background
Diagnosing sacroiliitis by magnetic resonance imaging (MRI) in children/adolescents can be difficult due to the growth-related changes. This study analyzed the normal osseous anatomy of the sacroiliac joints (SIJ) in a juvenile population using computed tomography (CT).
Methods
The anatomy of the SIJ was retrospectively analyzed in 124 trauma patients aged 9 months – <18 years by CT, based on 2 mm slices in axial, semi-axial and semi-coronal planes. The following anatomical features were recorded: intersegmental fusion of the sacral vertebral segments 1–3 (S1-S3), ossified nuclei (antero-superior at S1, lateral to the intervertebral spaces and lateral to S1 and S2) and joint facet defects larger than 3 mm.
Results
Fusion of S1/S2 started at the age of 6 years and was complete after the age of 13 years in most girls and after the age of 14 years in most boys. Fusion of S2/S3 started at the age of 9 years, but could remain incomplete up to 18 years in both genders. Ossified nuclei antero-lateral at S1 and/or in the joint space were observed until the age of 18 years and occurred in 77% of individuals ≥13 years with intraarticular localization in 64% of girls and 60% of boys. Joint facet defects >3 mm occurred in 21 children/adolescents (17%) located to both the iliac and sacral joint facets.
Conclusions
Normal osseous SIJ structures in children and adolescents vary considerably. Attention to these normal anatomical structures during growth may help to avoid false positive findings by MRI.
Background
The sacroiliac joints (SIJ) play an important role in spondyloarthritis as the diagnosis often is confirmed based on sacroiliitis by imaging. In adults the early diagnosis of sacroiliitis is currently based on magnetic resonance imaging (MRI) [1, 2]. Since 2009 bone marrow edema (BME), highly suggestive of sacroiliitis, has been part of the classifications criteria for adult SpA as defined by Assessment of SpondyloArthritis (ASAS) International Society [1]. Inflammatory changes in the sacroiliac joints is also frequent in juvenile spondyloarthritis (JSpA) [3, 4] but the classification or diagnostic criteria used in adults seems not to be applicable in juvenile patients [5]. One of the explanations for somewhat different sacroiliac joints changes in children/adolescents compared with adults is the persistence of growth related edematous changes and lack of a clear delineation of minor osseous structures by MRI; in juvenile patients this is often a big challenge for radiologists [6, 7]. Usually both active and chronic MRI findings are required to establish the diagnosis in children and adolescents and distinct knowledge about the complicated anatomy of SIJ is therefore important [8]. Computed tomography (CT) is superior to MRI for visualizing detailed osseous anatomy in addition to pathological structural lesions [9,10,11] and can display chronic osseous changes in sacroiliitis, but not signs of inflammatory activity [9, 12, 13]. However, due to exposure to ionizing radiation, CT is not commonly used as the diagnostic method for diagnosing SIJ changes, especially not in children and adolescents.
For both adults and children/adolescents, early and correct diagnosis of inflammatory changes in the SIJ is only possible with precise knowledge of the structural anatomy and developmental variation of SIJ. A literature review resulted in a few studies analyzing the anatomy of the SIJ based on CT in adults [10, 13,14,15]; two of these focused on prevalence and appearance of anatomical variants [14, 15]. In children and adolescents, CT evaluation was performed in one study with the purpose of evaluating developmental features. A total of 25 juvenile individuals were examined by single slice CT resulting in the detection of ossification centers in the SIJ in individuals aged >15 years [16].
The purpose of this study was to analyze the normal osseous anatomy of the SIJ in children and adolescents using current multislice CT technique.
Methods
Study population
The study was designed as a retrospective analysis conducted at the Department of Radiology, Aarhus University Hospital, Denmark.
The study population comprised patients selected from a local trauma database at our hospital between January 2012 and August 2016. A total of 194 juvenile patients met our trauma team activation criteria according to the international ABCDE-system having a score ≥ 2 [17] and the indication for total-body CT used by Treskes et al. in the REACT-2 trial [18]. They all underwent CT scanning encompassing the head, neck, chest, abdomen and pelvis. Inclusion criteria to our study were: Age below 18 years and no CT detectable traumatic changes in the spine, pelvis, abdomen and chest. Exclusion criteria: Traumatic skeletal and/or organ lesions in the chest, abdomen, pelvis and spine, changes in the SIJ suggestive of sacroiliitis, previous spine or pelvic surgery, CT reconstructions in a bony setting not including the whole SIJ (Fig. 1). In total, 124 patients aged 9 months – <18 years (mean age 11 years 8 months), 57 girls and 67 boys, were included in our study. One girl was examined twice: at the age of 13 years and at the age of 15 years.
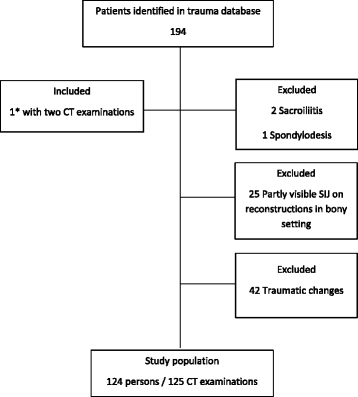
Flowchart of study population. * 1 girl included with two CT examinations being 13 and 15 years old
CT examination
All patients were examined in supine position using a 64-slice Philips Brilliance CT scanner (USA, Cleveland), according to the standard department guidelines. A post-contrast CT examination of the thorax, abdomen and pelvis was performed using a beam collimation of 0.625 mm, slice thickness of 2 mm, increment 1 mm, kV 100–140 and mAs 100–350 appropriately adjusted for patient size and shape. Axial spine reformats, including the SIJ, with a slice thickness of 1 mm and an increment of 0.5 mm, using a bony reconstruction filter D, were routinely performed and post-processed at a Philips IntelliSpace workstation with software version 6.0.4.02700 for reconstructions of the SIJ. Axial, semi-axial and semi-coronal 2 mm thick contiguous CT slices covering the entire SIJ were used for evaluation of the SIJ.
Evaluation of CT examination
The following anatomical features were recorded: 1) Intersegmental fusion of the sacral vertebral segments 1–3 (S1-S3) (Fig. 2), 2) ossified nuclei antero-superior at S1 (Fig. 3), 3) ossified nuclei lateral to the intersegmental spaces at S1/S2 and S2/S3 (Fig. 2), 4) ossified nuclei lateral to vertebral segment S1 and S2, respectively (Fig. 4), and 5) joint facet defects larger than 3 mm (Fig. 5).
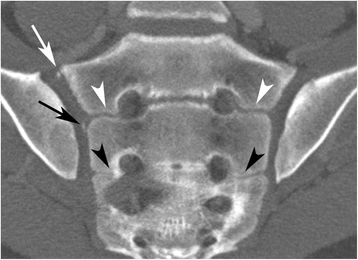
Fusion between sacral segments S1-S3 in a 14-year-old boy. Semi-coronal slice. Open space (no fusion) at the level S1/S2 – white arrowhead; partial fusion at the level S2/S3 – black arrowhead. Note also ossified nucleus antero-superior at S1 (white arrow) and lateral to the intervertebral space S1/S2 (black arrow) on the right side
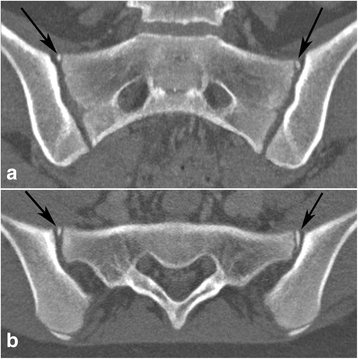
Ossified nuclei antero-superior at S1 in a 13-year-old girl. Bilateral ossified nuclei antero-superior at S1 - black arrows. a - semi-coronal and (b) - semi-axial slice
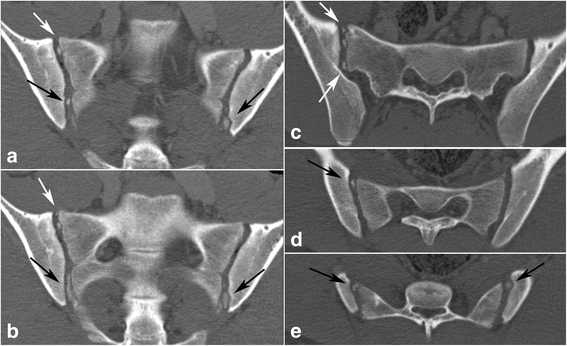
Multiple (≥ three) ossified nuclei in the joint space in a 16-year-old boy. Left images labeled (a), (b) – semi-coronal slices; right semi-axial images marked by (c) at the level of S1 and (d), (e) at the level of S2. White arrows – ossified nuclei lateral to the first sacral segment; black arrows – ossified nuclei lateral to the second sacral segment
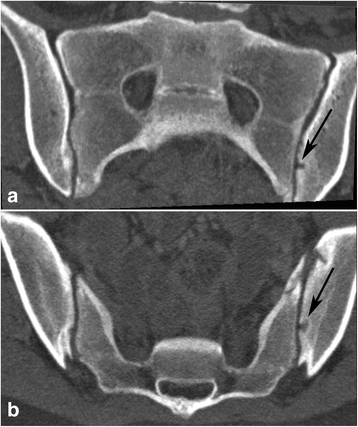
Joint facets defects ≥3 mm. 14-year-old girl with joint facets defect larger than 3 mm in the left iliac surface - black arrow; (a) - semi-coronal and (b) - semi-axial slice
Intersegmental fusion of sacral vertebrae S1 - S3 was characterized as absent when no osseous bridging was observed in the intersegmental space on all coronal slices. Partial fusion was reported in case of ossification in intersegmental spaces and complete fusion at the time of no detectable space between the sacral segments.
All 125 CT examinations of the SIJ were evaluated by a senior musculoskeletal radiologist (AZ). The reliability of the evaluations was assessed based on an independently evaluation of 48 examinations/96 SIJ by another senior radiologist (AGJ). Inter-observer agreement was based on these results.
Statistical analysis
The frequency of segmental fusion and occurrence of ossified nuclei were analyzed descriptively in relation to age and gender. Gender related developmental differences were analyzed using Pearson's chi-squared test or Fisher exact test. A level of p < 0.05 was considered significant.
Inter-observer variation regarding nuclei in the 96 joint spaces was assessed by Bland-Altman analysis using plots, bias and 95% confidence interval, and disagreements were subsequently analyzed to obtain consensus.
Results
The two radiologists agreed on intersegmental fusion except in two (4%) of the 48 individuals and disagreed on joint facet defects in three of the 192 (1,5%) articular surfaces. They agreed on the number of nuclei in the joint space in 85 of the 96 (88%) joints. The disagreement regarding the number of nuclei was low with a bias of 0.1 and a 95% confidence interval of 0.0–0.2.
Results of the age and gender distribution of intersegmental fusion of S1-S3, occurrence of ossified nuclei antero-superior at S1 and in the SIJ space, and joint facet defects are shown in Table 1.
Fusion of S1/S2 intersegmental space was not seen before the age of 6 years, and the frequency increased with age. Complete fusion was seen in one girl at 7 years and 10 months but predominantly occurred in girls after the age of 13; however, with remnants of space in one girl aged >16 years. Complete fusion was less common in boys occurring significantly less frequently in boys ≥14 years than in girls in the same age range (X 2 = 6.94, p = 0.008).
Fusion of the intersegmental space S2/S3 started at the age of 9 years, but there were remnants of space in both genders up to the age of 18 years. Complete fusion between S2 and S3 was observed 2 years earlier in girls than in boys, occurring in four of six girls aged 13–14 years and in three of seven boys aged 15–16 years, though not statistically significant.
Nuclei antero-superior at S1 and in the joint space rarely occurred before the age of 13 years, observed in three girls; two of them had both nuclei antero-superior at S1 and in the join space (in a 11-year-old girl lateral to S1 and in a 12-year-old girl lateral to S1 and at the level S1/S2, respectively). In individuals aged ≥13 years ossified nuclei at or in the joint space were frequent, occurring in 21 of 28 girls (75%) and in 26 of 33 boys (79%).
The antero-superior area at S1 was the most frequent location of ossified nuclei in individuals ≥13 years old, observed in 19 of 28 (68%) girls and 21 of 33 (64%) boys. The nuclei often occurred bilaterally and were visualized on both coronal and axial slices (Fig. 3). Nuclei at this location were first observed at the age of 10 years in a girl but were not seen in boys before the age of 13 years. Although they predominantly occurred in the age range 14 - ≤17 years, nuclei at this location were observed in two girls and three boys in the age range 17 - <18 years.
Intraarticular localization of ossified nuclei was seen in 18 of 28 (64%) girls and in 20 of 33 (60%) boys >13 years. In total, 25 of 61 persons (41%) aged ≥13 years had multiple (≥ three) ossified nuclei in the joint space, observed in 11 (39%) girls and 14 (42%) boys, respectively. Persistence of ≥ three nuclei located intraarticularly was observed in one girl and three boys ≥17 years old.
Joint facet defects over 3 mm were observed in 21 children (17%), 10 girls and 11 boys, occurring in all age groups. Bilateral defects occurred in one boy who had a total of three lesions (Fig. 5). Thirteen of the defects were localized to the iliac joint facet and ten to the sacral joint facet.
Discussion
The present study highlights the complex osseous anatomy of juvenile SIJs during skeletal growth. There is a frequent occurrence of ossified nuclei antero-lateral to S1 or in the joint space in children and adolescents over 13 years (77% of individuals ≥13 years in current study). The occurrence of joint facet defects over 3 mm was observed in all age groups, but in 21% of individuals ≥13 years. Both ossified nuclei and joint facet defects could be challenging for radiologists or rheumatologists assessing MRI regarding inflammatory changes as growth related changes may simulate inflammatory lesions.
Computed tomography of juvenile SIJs is rarely use in the diagnosis of sacroiliitis due to the exposure to ionizing radiation. Review of the literature resulted in one study where 25 children/adolescents were examined by CT for unknown reasons. A total of seven individuals aged 15–19 years had ossified nuclei in the SIJ, all apparently occurring at the first sacral segment. The age of the remaining individuals in the study was not stated [16]. Another age range than in the present study may have influenced the lower frequency of nuclei compared to our detection of ossified nuclei in 50 of 125 (40%) individuals including nuclei in the joint space in 40 (32%) individuals. In the current study, ossified nuclei were observed 5 years earlier than in the study by Götz et al. [16] with the occurrence of ossified nuclei antero-lateral at S1 in one 10-year-old girl. We also detected nuclei more distally located in the joint space. Such nuclei were not detected by Götz et al. at CT, but their histological analyses of three autopsy specimens revealed the presence of nuclei at the level of the second and third sacral segment. It is therefore possible that the different scan techniques may influence results. The present use of 2 mm slices in three different reconstruction planes ought to increase the detectability of nuclei compared to axial 4–8 mm slices used in the study by Götz et al. [16].
The SIJ anatomy has also been evaluated by MRI [6]. Based on 114 individuals aged 8–17 years, findings regarding intersegmental fusion were rather similar to our observations with fusion occurring earlier in girls than in boys. Apophyses/nuclei lateral to S1 or at the intersegmental space S1/S2 were also observed between the age of 9 to 17 years. This is in accordance with the present findings except that Bollow et al. found complete ossification and apparently fusion of the nuclei at S1 in the 25 oldest children, mean age 15.9 ± 1.3 years. In the present study persistence of nuclei was also observed in the age group 17- < 18 years, in both girls and boys. The difference is probably due to a better delineation of minor osseous structures by CT than by MRI.
Growth-related changes can lead to false positive MRI results regarding both activity and structural lesions. Recent findings have revealed a limited specificity of BME alone to define a positive MRI for SpA in both children/adolescents and adults [19, 20]. Subtle non-specific BME at the SIJ suggestive of sacroiliitis was reported in 20% of a juvenile control group as well as in 21% of adult patients with low back pain (LBP) [20, 21]. The causes of subchondral non–inflammatory BME at the SIJ in juvenile subjects have not yet been analyzed. However, the high prevalence of ossified nuclei in the joint space in children and adolescents over 13 years could be the cause of the observed BME in 20% of the juvenile control subjects with a mean age ± SD of 15;1 ± 2;3 years [20]. An increased bone turn-over during ossification of cartilage-preformed bone could be a possible explanation. The ossified nuclei detected in the current study are probably located in the sacral cartilage, as shown histopathologically in three autopsy specimens [16]. The process resulting in fusion of the ossified nuclei to the main ossified sacral segment thus corresponds to the growth process occurring as part of epiphyseal cartilage fusion in tubular bones where the process is accompanied by BME. It has to our knowledge not been evaluated whether or not BME in healthy juvenile persons predominantly occur in the sacrum.
The presence of erosions was found to be a rather specific MRI feature for sacroiliitis in juvenile SpA patients, as it was reported to be present in 58–96% of patients [8, 20]. However, erosions by MRI of the SIJ were reported in 9% of a juvenile control group as well as in 11% of adults with non-inflammatory LBP [20, 21]. Defining erosion by MRI may be difficult due to a poor visualization of cortical bone in the widely used T1-weighted sequences and a high variability of the appearance of erosions. This is supported by moderate inter-observer agreement in juvenile individuals as well as in adults [20, 22]. Several studies evaluating the value of CT in the diagnosis of sacroiliitis in adults have shown higher specificity of CT for detection of erosions and subchondral sclerosis compared to radiography and the commonly used T1-weighted MR-images [11,12,13, 23,24,25]. Similar analyses have not been performed in juvenile populations to avoid radiation exposure by CT. Radiation disadvantages by CT have also been a limiting factor for assessing CT morphology in the healthy adult population. However, a study of 45 asymptomatic adults revealed bilateral erosions in only one case, indicating erosions to be rather specific signs of sacroiliitis [26]. In our study, irregularity of the osseous margins of the iliac and/or sacral articular surface with articular defects over 3 mm occurred in 17% and may simulate erosions by MRI.
One of the strengths of the current study is the wide age distribution illustrating the anatomical variety of the juvenile SIJ. Another strength is a uniform study recruitment and the standardized CT technique including reconstructions.
Some limitations need to be considered. The number of persons included in the analysis was rather small, resulting in few representatives among girls and boys in some of the age groups. Thus, our results must be interpreted with some caution. Only one reader evaluated all SIJ examinations, but the inter-observer agreement was good.
Knowledge of osseous anatomy at the SIJ is crucial to make a correct early diagnosis in children/adolescents, especially considering the appearance of structural changes in the early phase of SpA [27,28,29]. Further studies in a larger population and analyses of the correlation between CT and MRI appearances of structural findings in SIJ would be optimal but probably not feasible. A prospective study with MRI supplementing a CT examination performed on a clinical indication such as suspicious traumatic changes can be a possibility, though difficult to perform. However, the results of the current study could be used to define the anatomy of the SIJ by MRI in clinical practice. The possible presence of subchondral BME in relation to ossified nuclei in the articular cartilage needs to be investigated. An evaluation of SIJ anatomy in healthy juvenile persons using dedicated cartilage sequences and high-resolution 3 T image quality can potentially visualize the complex anatomy and explain BME distribution on concomitant STIR or T2- weighted fat suppressed sequences.
Conclusions
Normal osseous SIJ structures in children and adolescents vary considerably. Intraarticular ossified nuclei are frequent after the age of 13 years and can persist up to the age of 18 years in both genders. Attention to normal anatomical structures during growth may help to avoid false positive MRI findings.
Abbreviations
- ASAS:
-
Assessment of SpondyloArthritis International Society
- BME:
-
Bone marrow edema
- CT:
-
Computed tomography
- JSpA:
-
Juvenile Spondyloarthritis
- LBP:
-
Low back pain
- MRI:
-
Magnetic resonance imaging
- SIJ:
-
Sacroiliac joints
- SpA:
-
Spondyloarthritis
References
-
Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–7.
-
Weber U, Zubler V, Pedersen SJ, Rufibach K, Lambert RG, Chan SM, et al. Development and validation of a magnetic resonance imaging reference criterion for defining a positive sacroiliac joint magnetic resonance imaging finding in spondyloarthritis. Arthritis Care Res (Hoboken). 2013;65:977–85.
-
Hussein A, Abdul-Khaliq H, von der Hardt H. Atypical spondyloarthritis in children: proposed diagnostic criteria. Eur J Pediatr. 1989;148:513–7.
-
Strom H, Lindvall N, Hellstrom B, Rosenthal L. Clinical, HLA, and roentgenological follow up study of patients with juvenile arthritis: comparison between the long term outcome of transient and persistent arthritis in children. Ann Rheum Dis. 1989;48:918–23.
-
Herregods N, Dehoorne J, Van den Bosch F, Jaremko JL, Van Vlaenderen J, Joos R, et al. ASAS definition for sacroiliitis on MRI in SpA: applicable to children? Pediatr Rheumatol Online J. 2017;15:24-017–0159.
-
Bollow M, Braun J, Kannenberg J, Biedermann T, Schauer-Petrowskaja C, Paris S, et al. Normal morphology of sacroiliac joints in children: magnetic resonance studies related to age and sex. Skelet Radiol. 1997;26:697–704.
-
Weiss PF, Xiao R, Biko DM, Chauvin NA. Assessment of Sacroiliitis at diagnosis of juvenile Spondyloarthritis by radiography, magnetic resonance imaging, and clinical examination. Arthritis Care Res (Hoboken). 2016;68:187–94.
-
Herregods N, Dehoorne J, Joos R, Jaremko JL, Baraliakos X, Leus A, et al. Diagnostic value of MRI features of sacroiliitis in juvenile spondyloarthritis. Clin Radiol. 2015;70:1428–38.
-
Devauchelle-Pensec V, D'Agostino MA, Marion J, Lapierre M, Jousse-Joulin S, Colin D, et al. Computed tomography scanning facilitates the diagnosis of sacroiliitis in patients with suspected spondylarthritis: results of a prospective multicenter French cohort study. Arthritis Rheum. 2012;64:1412–9.
-
Geijer M, Gadeholt Gothlin G, Gothlin JH. The validity of the New York radiological grading criteria in diagnosing sacroiliitis by computed tomography. Acta Radiol. 2009;50:664–73.
-
Puhakka KB, Jurik AG, Egund N, Schiottz-Christensen B, Stengaard-Pedersen K, van Overeem HG, et al. Imaging of sacroiliitis in early seronegative spondylarthropathy. Assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003;44:218–29.
-
Hu L, Huang Z, Zhang X, Chan Q, Xu Y, Wang G, et al. The performance of MRI in detecting subarticular bone erosion of sacroiliac joint in patients with spondyloarthropathy: a comparison with X-ray and CT. Eur J Radiol. 2014;83:2058–64.
-
Tuite MJ. Sacroiliac joint imaging. Semin Musculoskelet Radiol. 2008;12(1):72–82.
-
Demir M, Mavi A, Gumusburun E, Bayram M, Gursoy S, Nishio H. Anatomical variations with joint space measurements on CT. Kobe J Med Sci. 2007;53:209–17.
-
Prassopoulos PK, Faflia CP, Voloudaki AE, Gourtsoyiannis NC. Sacroiliac joints: anatomical variants on CT. J Comput Assist Tomogr. 1999;23:323–7.
-
Gotz W, Funke M, Fischer G, Grabbe E, Herken R. Epiphysial ossification centres in iliosacral joints: anatomy and computed tomography. Surg Radiol Anat. 1993;15:131–7.
-
Kool D, Blickman JG. Advanced trauma life support. ABCDE from a radiological point of view. Emerg Radiol. 2007;14:135–41.
-
Treskes K, Bos SA, Beenen LFM, Sierink JC, Edwards MJR, Beuker BJA, et al. High rates of clinically relevant incidental findings by total-body CT scanning in trauma patients; results of the REACT-2 trial. Eur Radiol. 2017;27:2451–62.
-
Arnbak B, Jensen TS, Egund N, Zejden A, Horslev-Petersen K, Manniche C, et al. Prevalence of degenerative and spondyloarthritis-related magnetic resonance imaging findings in the spine and sacroiliac joints in patients with persistent low back pain. Eur Radiol. 2016;26:1191–203.
-
Jaremko JL, Liu L, Winn NJ, Ellsworth JE, Lambert RG. Diagnostic utility of magnetic resonance imaging and radiography in juvenile spondyloarthritis: evaluation of the sacroiliac joints in controls and affected subjects. J Rheumatol. 2014;41:963–70.
-
Arnbak B, Grethe Jurik A, Horslev-Petersen K, Hendricks O, Hermansen LT, Loft AG, et al. Associations between Spondyloarthritis features and magnetic resonance imaging findings: a cross-sectional analysis of 1,020 patients with persistent low back pain. Arthritis Rheumatol. 2016;68:892–900.
-
Arnbak B, Jensen TS, Manniche C, Zejden A, Egund N, Jurik AG. Spondyloarthritis-related and degenerative MRI changes in the axial skeleton--an inter- and intra-observer agreement study. BMC Musculoskelet Disord. 2013;14:274.
-
Kozin F, Carrera GF, Ryan LM, Foley D, Lawson T. Computed tomography in the diagnosis of sacroiliitis. Arthritis Rheum. 1981;24:1479–85.
-
Carrera GF, Foley WD, Kozin F, Ryan L, Lawson TL. CT of sacroiliitis. AJR Am J Roentgenol. 1981;136:41–6.
-
Yu W, Feng F, Dion E, Yang H, Jiang M, Genant HK. Comparison of radiography, computed tomography and magnetic resonance imaging in the detection of sacroiliitis accompanying ankylosing spondylitis. Skelet Radiol. 1998;27:311–20.
-
Vogler JB 3rd, Brown WH, Helms CA, Genant HK. The normal sacroiliac joint: a CT study of asymptomatic patients. Radiology. 1984;151:433–7.
-
Weber U, Jurik AG, Lambert RG, Maksymowych WP. Imaging in Spondyloarthritis: controversies in recognition of early disease. Curr Rheumatol Rep. 2016;18:58.
-
Weber U, Lambert RG, Ostergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62:3048–58.
-
Althoff CE, Sieper J, Song IH, Haibel H, Weiss A, Diekhoff T, et al. Active inflammation and structural change in early active axial spondyloarthritis as detected by whole-body MRI. Ann Rheum Dis. 2013;72:967–73.
Acknowledgements
Not applicable.
Funding
There were no grants or financial supports of the study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article and revising it critically for important intellectual content, and all authors approved the final version to be published. Study conception and design: AGJ, AZ. Acquisition of data: AZ, AGJ. Analysis and interpretation of data: AZ, AGJ.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study complied with the Helsinki Declaration and was approved by the Central Denmark Region Committee on Health Research Ethics (1–10–72-148-14) and the Danish Data Protection Agency (1–16–02-7-15).
Consent for publication
Not applicable
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Zejden, A., Jurik, A.G. Anatomy of the sacroiliac joints in children and adolescents by computed tomography. Pediatr Rheumatol 15, 82 (2017). https://doi.org/10.1186/s12969-017-0210-0
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12969-017-0210-0
Keywords
- Sacroiliac joints
- Computed tomography
- Juvenile individuals
- Anatomy
- Sacroiliitis
- Juvenile spondyloarthritis
Source: https://ped-rheum.biomedcentral.com/articles/10.1186/s12969-017-0210-0
0 Response to "Sacroiliac Joint Issues in Pediatrics Continuing Education"
Post a Comment